CASE REPORT | https://doi.org/10.5005/jp-journals-10023-1182 |
Post-Tubercular Upper Airway Stenosis: Our Experience
1Department of ENT, Sushrut Institute of Plastic Surgery and Super-specialty Hospital, Lucknow, Uttar Pradesh, India
2Department of ENT, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
3Department of ENT, Nairobi Hospital, Nairobi, Kenya
Corresponding Author: Ashish C Agarwal, Department of ENT, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India, Phone: +91 9663558322, e-mail: ashishagr3@rediffmail.com
How to cite this article Srivastava R, Agarwal AC, Macharia IM. Post-Tubercular Upper Airway Stenosis: Our Experience. Int J Phonosurg Laryngol 2020;10(1):25–28.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Aim: This study describes the findings seen in patients suffering from stenosis of the upper airway secondary to tuberculosis (TB) and its management.
Materials and methods: A prospective study was done at a tertiarycare hospital between August 2017 and April 2019 on four patients diagnosed with airway stenosis secondary to TB.
Results: There were three males and one female, and the age ranged from 18 to 38 years. Rechanneling of the stenosed segment was done using cold instruments and CO2 laser, used alone or in combination. One patient developed recurrence even after undergoing two procedures which had to be eventually treated by resection anastomosis.
Conclusion: Early diagnosis and treatment with antitubercular medications is the key to prevent the development of airway stenosis. The treatment of stenosis involves surgical intervention using cold instruments and laser, and the chances of getting satisfactory results are good. Regular follow-up of such patients is extremely important.
Clinical significance: Development of airway stenosis secondary to TB is a dreaded sequelae of the disease pathogenesis. This entity should be kept as one of the differential diagnoses while evaluating patients with upper airway stenosis in whom the etiology of the condition remains elusive.
Keywords: Airway stenosis, CO2 laser, Tuberculosis..
INTRODUCTION
Tuberculosis (TB) is a major health problem in India. It is transmitted through inhalational route, and the lung is the commonest organ to be affected. According to the World Health Organization (WHO) statistics of 2011, the estimated load of the disease is 2.2 million cases in India out of a global burden of 9.6 million.1 In India, each year approximately 220,000 deaths are reported due to TB. Between 2006 and 2014, the disease had cost the Indian economy around 340 billion USD.1 The incidence of TB is rising in India due to multidrug resistant organisms, deteriorating healthcare infrastructure, and overcrowding. The pattern of laryngeal tuberculosis is also changing.
The incidence of laryngeal TB is less than 1% of all TB cases.2 Upper airway involvement by the disease is becoming common in India. Healing of tubercular lesion in the upper airway leads to stenosis. Tuberculosis of the larynx may be primary or secondary to a pulmonary lesion. The pathophysiology of upper or lower airway stenosis secondary to tuberculosis is similar. The changes initially begin with infiltration of the bacilli into the mucosa and submucosa which later leads to formation of ulcers, granulation tissue, fibrosis, and in the end an organized stenosis. In trachea and bronchus, the inflammatory changes have lymphocyte infiltration that later develop into caseous necrosis. The necrotic area forms deep craters that later turn into granulation tissue (hypertrophic inflammatory polyps). In advanced stages, fibrous hyperplasia and contracture develops leading to stenosis. The incidence of airway stenosis may be up to 68% in the initial months3,4 which rises with prolongation of the disease.5 The present article describes our experience as a case series of four patients of upper airway stenosis secondary to tuberculosis. None of the patient was immune-compromised.
MATERIALS AND METHODS
A study was done on patients visiting the outpatient ear, nose, and throat (ENT) department of a tertiary-care hospital in north India between August 2017 and April 2019, with complaints of cough, throat pain, breathing/swallowing difficulty, voice change, sputum production, fever, and weight loss. The patients were evaluated by a clinical examination, followed by throat endoscopy, blood investigations, radiological examination (X-ray/CT scan of neck and thorax or both), sputum microscopy/culture, and Mantoux test. A histopathological examination of the tissue sample was done if required. The patients diagnosed with TB were included in the study. The patients with a past history of a surgical procedure in the throat or a history of trauma to the neck were excluded. The objectives of this study were to describe the intraoperative findings, the surgical technique used, the complications encountered, and the postoperative results. An informed consent was obtained from the patients, and an ethical clearance was obtained from the institutional ethics committee.
Case 1
The first case was a 21-year-old gentleman who presented with difficulty in breathing for the past 3 months which had aggravated in the last 3 days. He had been treated for pulmonary tuberculosis and the same had completed 3 months prior to presentation. A flexible laryngo-bronchoscopic examination revealed stenosis at the level of second/third tracheal ring with two very small openings, multiple webs, and secretions (Fig. 1). After an informed consent and planning with an anesthetist, the patient was taken up for surgical correction. The surgery was done under total intravenous anesthesia (TIVA) with propofol and fentanyl infusion, and laryngeal mask airway (LMA) of size 3 was used. Through the side channel of LMA, a flexible bronchoscope of size 3.5 mm was inserted. The bronchoscope’s working channel was used for delivering 400-micron diode laser fiber.
At the stenosed segment, radial incisions were given at 12-, 3-, and 9-o’clock position. Once a sufficient opening of the airway (around 4–5 mm) was obtained, a suspension laryngoscope was placed. Bougie dilatation with 7/9 mm was done (Fig. 2). Intermittent apnea technique (tube in–tube out technique) was employed during the procedure. After dilatation, 2 mg/mL of Mitomycin-C was applied, and injection triamcinolone acetonide was injected at the periphery of the stenosed site. The patient was discharged the next day with an advice of twice-daily nebulization with budesonide (0.5 mg), ipratropium (500 μg), and levosalbutamol (1.25 mg) for 3 weeks. He was followed regularly at an interval of 3 to 4 weeks in the outpatient department. Flexible scopy of the airway was done at each visit. After 4 months, the patient presented again with difficulty in breathing following an attack of upper respiratory infection. Flexible scopy showed circumferential tracheal stenosis covered with slough and secretions. The abovementioned procedure was repeated, and same follow-up was done. The patient again developed similar symptoms for the third time after 5 months. This time resection anastomosis (involving 4 tracheal rings) was done as a single-stage procedure, and the postoperative period was uneventful. The patient has been on regular follow-up and has remained asymptomatic till date.
Case 2
An 18-year-old boy presented with breathing difficulty for the past 3 months. He had developed change in voice and painful swallowing in the last 2 years. He was diagnosed to have laryngeal tuberculosis and had taken antitubercular treatment (ATT) for 9 months. Airway assessment was done with a flexible scope. There was an anterior glottis web and around 2-mm chink of airway. Emergency tracheostomy was performed followed by airway assessment under inhalational anesthesia. Posterior glottic stenosis with immobile cricoarytenoid joint was found (Fig. 3). CO2 laser-assisted release of the web along with silicon keel placement and posterior cordotomy with partial arytenoidectomy (Fig. 4) was carried out. The laryngeal keel was removed after 3 weeks. The patient was gradually decannulated after 6 weeks of surgery. The patient is under follow-up after 2 years of surgery and remains asymptomatic.

Fig. 1: Lesion in the first case showing tracheal stenosis (2 key holes with web and discharge)
Case 3
A 38-year-old female presented with change in voice of 4 months duration and noisy breathing during sleep. The patient had a past history of taking ATT for 9 months. Airway assessment was done with a flexible scope, and supraglottic and glottic narrowing having a lumen of around 6 mm was seen (Fig. 5). The stenosis was released using a CO2 laser. A silicon sheet (1 mm thick) was placed as a stent (Fig. 6). The same was removed after 3 weeks. The patient was followed up for 1 year; she had a satisfactory voice and no breathing difficulty.
Case 4
A 24-year-old male patient presented for a follow-up visit. He had a history of cough, fever, and loss of appetite 2 years back and was diagnosed with pulmonary tuberculosis. Following this, he was started on ATT. After 9 months of ATT, the patient had developed stridor, a tracheostomy was done, and eventually the patient had completed 12 months of ATT. During the current visit, he complained of a change in voice. A flexible endoscopy of the airway showed an anterior glottic web. He was taken up for laser-assisted release of the web and keel placement. Intraoperatively, the micro-laryngoscopy examination under TIVA showed anterior and posterior glottic stenosis (Fig. 7). The case was managed in a similar way as the previous mentioned patient (case 2).
DISCUSSION
Upper airway involvement due to TB is either due to an air-borne spread or secondary due to implantation of the organism from infected sputum or hematogenous dissemination or infection via lymphatics.6 The pathogenesis of upper airway stenosis secondary to tuberculosis is same as endobronchial (lower airway) stenosis. The pathological changes involve the mucosa, submucosa, and eventually the cartilage. The end result is fibrosis and stenosis of the airway. Endobronchial and tracheal TB affect the cartilage framework. There are various barriers in the form of muscle and fat planes in the upper airway which makes it less prone to involvement by the disease. TB bacilli affect both the surfaces of the vocal folds and there is hyperemia, ulcers, and granulation tissue formation. These lesions respond to ATT and might heal with the formation of a web and sometimes involvement of the crico-arytenoid joint.

Fig. 2: Site of the lesion in the first case after using laser and bougie dilatation
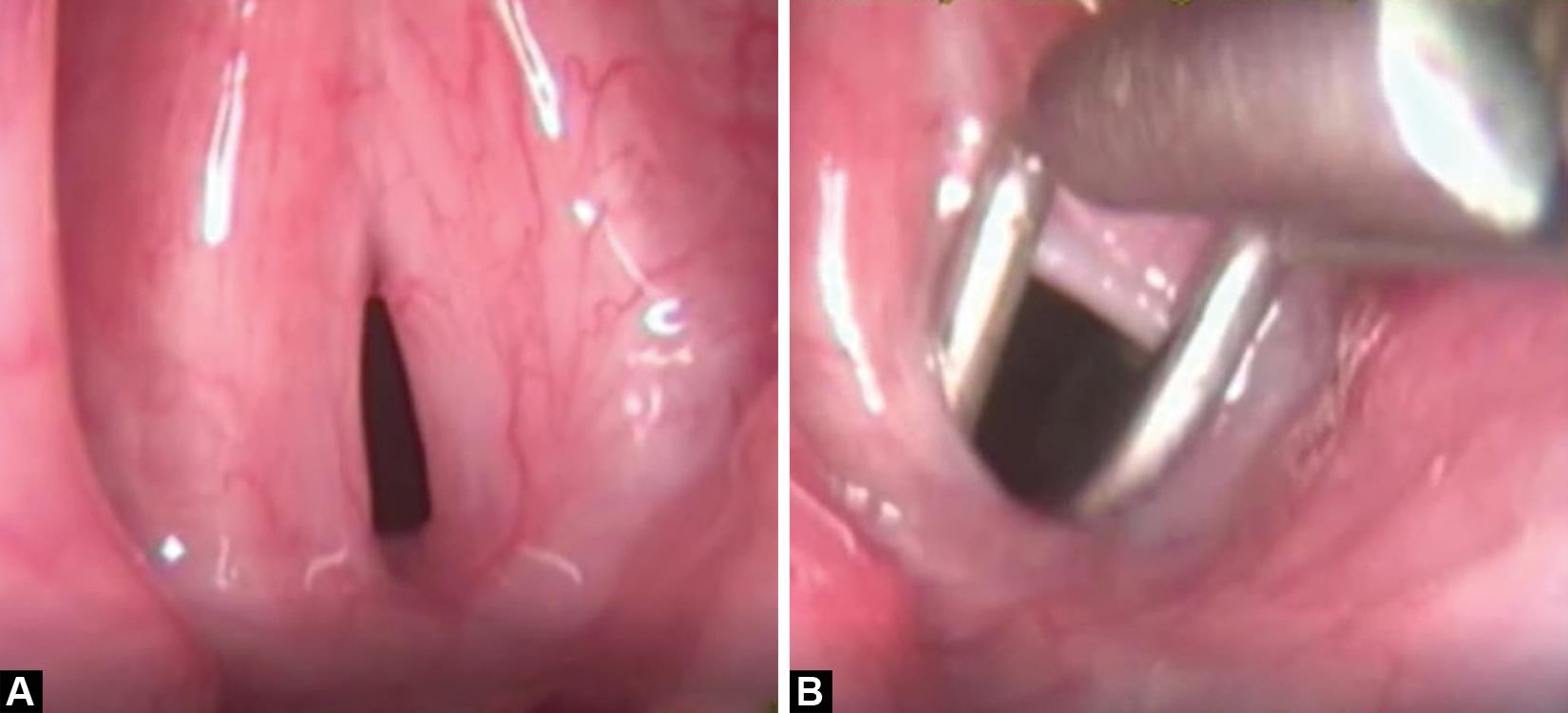
Figs 3A and B: Endoscopic picture of the second patient showing: (A) Anterior glottic web; (B) Immobile cricoarytenoid joint

Fig. 4: Intraoperative photograph of the second patient demonstrating a silicon keel after the glottic web release and posterior cordotomy
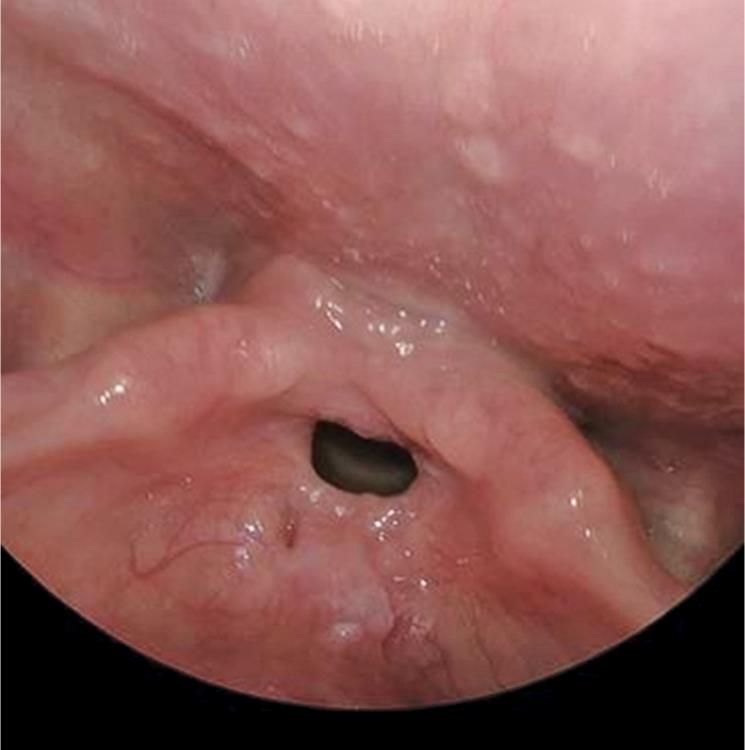
Fig. 5: Supraglottic and glottic stenosis in the third case

Fig. 6: The silicon sheet placed after laser assisted release in the third case

Fig. 7: Endoscopic photograph demonstrating the anterior and posterior glottic stenosis in the fourth case
Patients with primary laryngeal tuberculosis have a normal chest X-ray. Primary laryngeal involvement is seen in about 19% of TB cases.7 Laryngeal tuberculosis maybe categorized into four groups, namely, (a) whitish ulcerative lesions (40.9%), (b) nonspecific inflammatory lesions (27.3%), (c) polypoid lesions (22.7%), and (d) ulcerofungative mass lesions (9.1%).8 In a recent publication on 15 cases of laryngeal tuberculosis by Marina Saldanha et al.,9 two patients had primary laryngeal tuberculosis, and the others had an associated pulmonary involvement. Change in voice was the most common complaint, and ulcerative lesions were the most common finding. In our study, one of four cases had a primary laryngeal lesion (case 2).
Earlier studies have shown that TB mainly involves the posterior larynx.10 The recent studies point toward a predilection for vocal cords (50–70%) followed by false cords (40–50%), epiglottis, aryepiglottic folds, arytenoids, posterior commissure, and subglottis (10–15%).11 Lower airway can also be affected by tuberculosis. Tracheobronchial involvement was first described by Richard Morton, an English Physician in 1698.12 Lower airway involvement evolves from submucosal ulceration to necrosis with subsequent healing leading to a circumferential long segment stenosis. Cases 2 and 4 of our study presented with anterior and posterior glottis stenosis.
Case 1 presented with tracheal stenosis and fibrinous secretions. It recurred after the initial treatment due to perichondritis. Tracheo-bronchial stenosis due to TB is resistant to medical treatment and requires surgical intervention. There are various procedures for managing bronchial stenosis, including. dilatation, stenting, fiber-guided laser, or cryotherapy. The incidence of restenosis after balloon dilatation is about 37.5%.13
Predictors of persistent airway stenosis in patients with endobronchial TB were studied by Um et al. on 67 patients.14 Persistent bronchial stenosis occurred in 41.8% of the patients. Advanced age of the patient and long duration of complaints before the start of ATT are predictors of bronchial stenosis. Oral steroids do not reduce the frequency of stenosis. So early diagnosis and treatment with ATT not only prevent the deeper effects on the airway but also reduce the unwanted sequel of bronchial stenosis. There are many reports in the literature on tracheal and bronchial stenosis secondary to TB,3 but there are few reports of laryngeal stenosis due to TB. A case report by Almeyda et al. affected only the subglottis.15 Our study would contribute to the existing knowledge about this topic.
CONCLUSION
There are few reports in the literature on glottic and supra-glottic stenosis due to tuberculosis. Any patient with supra-glottic or glottic stenosis of unknown cause should be investigated for tuberculosis. In such cases, history of taking ATT and old chest X-rays with findings suggestive of sarcoidosis, Wegener granulomatosis, amyloidosis,s and relapsing polychondritis help in reaching a logical conclusion.
CLINICAL SIGNIFICANCE
An early diagnosis and treatment with ATT not only prevent the deeper effects of tuberculosis on the airway but also reduce the unwanted sequel of bronchial stenosis. Advanced age and long duration of complaints before the start of ATT increase the chance of development of airway stenosis. Oral steroids do not have much role in reducing the stenosis. Surgical intervention with limited use of laser, placement of stent, and application of anti-proliferative agents have a definite role to play in the management of airway stenosis. Sometimes multiple surgeries are required to achieve optimum results. Regular follow-up of the patients post-intervention is important to diagnose a recurrence and planning of subsequent management.
REFERENCES
1. TB Facts 201, TB Statistics for India, Viewed 3 April 2013, %3C; http://www.tbfacts.org/tb-statistics-india.html%3E;.
2. Egeli E, Oghan F, Alper M, et al. Epiglottic tuberculosis in a patient treated with steroids for Addison′s disease. Tohoku J Exp Med 2003;201(2):119–125. DOI: 10.1620/tjem.201.119.
3. Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest 2000;117(2):385–392. DOI: 10.1378/chest.117.2.385.
4. Medlar EM. The behavior of pulmonary tuberculous lesions; a pathological study. J Lancet 1956;76(4):82–88.
5. Xue Q, Wang N, Xue X, et al. Endobronchial tuberculosis: an overview. Eur J Clin Microbiol Infect Dis 2011;30(9):1039–1044. DOI: 10.1007/s10096-011-1205-2.
6. Gandhi S, Kulkarni S, Mishra P, et al. Tuberculosis of larynx revisited: a report on clinical characteristics in 10 cases. Indian J Otolaryngol Head Neck Surg 2012;64(3):244–247. DOI: 10.1007/s12070-011-0333-4.
7. Harney M, Hone S, Timon C, et al. Laryngeal tuberculosis: an important diagnosis. J Laryngol Otol 2000;114(11):878–880. DOI: 10.1258/0022215001904220.
8. Shin JE, Nam SY, Yoo SJ, et al. Changing trends in clinical manifestations of laryngeal tuberculosis. Laryngoscope 2000;110(11):1950–1953. DOI: 10.1097/00005537-200011000-00034.
9. Saldanha M, Sima NH, Bhat VS, et al. Present scenario of laryngeal tuberculosis. Int J Otorhinolaryngol Head Neck Surg 2018;4(1):242–246. DOI: 10.18203/issn.2454-5929.ijohns20175633.
10. Kandiloros DC, Nikolopoulos TP, Ferekidis EA, et al. Laryngeal tuberculosis at the end of the 20th century. J Laryngol Otol 1997;111(70):619–621. DOI: 10.1017/s0022215100138137.
11. Ling L, Zhou SH, Wang SQ. Changing trends in the clinical features of laryngeal tuberculosis: a report of 19 cases. Int J Infect Dis 2010;14(3):230–235. DOI: 10.1016/j.ijid.2009.05.002.
12. Hudson EH. Respiratory tuberculosis: Clinical diagnosis Head ERG, ed. Symposium on tuberculosis. London: Cassell and Co; 1957. pp. 321–464.
13. Shahzad T, Irfan M. Endobronchial tuberculosis- a review. J Thorac Dis 2016;8(12):3797–3802. DOI: 10.21037/jtd.2016.12.73.
14. Um SW, Yoon YS, Lee SM, et al. Predictors of persistent airway stenosis in patients with endobronchial tuberculosis. Int J Tuberc Lung Dis 2008;12(1):57–62.
15. Almeyda J, Tolley NS, Ghufoor K, et al. Subglottic stenosis secondary to tuberculosis. Int J Clin Pract 1997;51(6):402–403.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.