ORIGINAL ARTICLE | https://doi.org/10.5005/jp-journals-10023-1180 |
Proposal of a Classification for Sulcus Following Microlaryngoscopy with a Retrospective Study of the Results of a Laser-assisted Sulcus Release Surgery
1–3Bombay Hospital Voice and Swallowing Centre, Bombay Hospital and Medical Research Centre, Mumbai, Maharashtra, India
Corresponding Author: Nupur K Nerurkar, Bombay Hospital Voice and Swallowing Centre, Bombay Hospital and Medical Research Centre, Mumbai, Maharashtra, India, Phone: +91 9821034085, e-mail: nupurkapoor@yahoo.com
How to cite this article Nerurkar NK, Nagree Z, Agrawal D. Proposal of a Classification for Sulcus Following Microlaryngoscopy with a Retrospective Study of the Results of a Laser-assisted Sulcus Release Surgery. Int J Phonosurg Laryngol 2020;10(1):3–8.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Background: Sulcus vocalis has historically remained challenging in terms of both diagnosis and management. The problem is further compounded by a lack of clarity in its classification as an accurate estimation of its presence and depth can be established only during microlaryngoscopy (MLS). We have thus proposed a classification of sulcus following MLS, which would allow for uniform grading. A laser-assisted sulcus release (LASR) technique is described which was performed in 7 patients and 10 vocal folds of isolated sulcus cases with outcome measures studied retrospectively.
Materials and methods: The classification proposed of sulcus following MLS is based on depth of invagination of the sulcus, length of the sulcus, presence of keratin debris within, and associated presence of mucosal bridges, polyps and cysts. A LASR technique was performed for type A and D sulci (based on proposed classification) where the multiple points of release were made with a CO2 laser Acublade perpendicular to the lips of the sulcus. All patients received pre- and postoperative voice therapy. Preoperative and 3 months’ postoperative stroboscopy and vocal outcome measures were reviewed retrospectively.
Results: Stroboscopic improvement of the mucosal wave and amplitude with decreased phonatory gap and decreased ventricular hyperadduction was observed in all patients postoperatively. There was a significant improvement of grade, roughness, breathiness, asthenia, strain (GRBAS) and maximum phonatory time (MPT) (p < 0.05).
Conclusion: The LASR technique performed for type A and D sulci in our limited case series revealed 3-month postoperative stroboscopic improvement along with improvement in vocal parameters. We plan to continue this study to include a larger sample size.
Keywords: CO2 laser, Laser surgery, Sulcus surgery, Sulcus vocalis..
INTRODUCTION
The relatively rare anatomical alteration of vocal folds called sulcus vocalis (SV), was first described in 1892 by Giacomini.1 Sulcus vocalis is a fibroplastic anomaly of the normally pliable vocal fold cover, where there is a migration of the vocal fold epithelium into the normally convex superficial lamina propria (SLP) or deeper, parallel to its free edge.2 It may be unilateral or bilateral and often varies in length and depth.
According to the literature, the frequency of occurrence of SV varies between 0.4 and 48%.3 This wide range of incidence is probably due to difficulty in identification of the superficial sulci even with stroboscopy and misdiagnosis. Various etiologies of sulci have been proposed, where some authors consider it to be of congenital origin,4 with a postulation of faulty genesis of the fourth and sixth branchial arches5 or a degeneration of fibroblasts in the macula flavae similar to age-related degeneration of vocal folds,6 while some have even suggested a familial occurrence.7 Nakayama et al. suggested an acquired origin,8 such as following chronic upper respiratory tract infection,9 phonotrauma,10 or rupture of vocal cyst.11 It has also been speculated that both factors play a role.12
Patients with SV usually present with hoarseness of voice, effortful voice production, easy vocal fatigability, reduced intensity, and raised pitch. On stroboscopic examination, a phonatory gap is often observed described as a spindle-shaped phonatory gap,13 which may be an asymmetrical spindle.14 Mucosal wave on stroboscopy may be reduced or absent, often medial furrows are better visualized during inspiration, and signs of supraventricular hyperfunction may be observed.15
Multiple classification systems have been described for sulcus depending on its morphology. In 1985, SV was classified by Bouchayer et al.5 followed a few years later by Nakayama et al.8 In 1996, Ford et al.15 proposed a classification followed by Pontes et al. in 2010.16 However, superficial sulci and focal pits may often be missed on stroboscopy and are often picked up only during examination and palpation of the vocal folds under general anesthesia (Figs 1 and 2). Often this incidental pickup is during surgery being performed for vocal fold cysts or polyps. Keeping this in mind, we have proposed a new classification system for sulcus based on findings under general anesthesia with microscopic magnification, palpation, and subepithelial infiltration. It is feasible to precisely diagnose and document superficial sulci, focal pit, exact depth of the sulcus, and presence of mucosal bridges in this fashion.
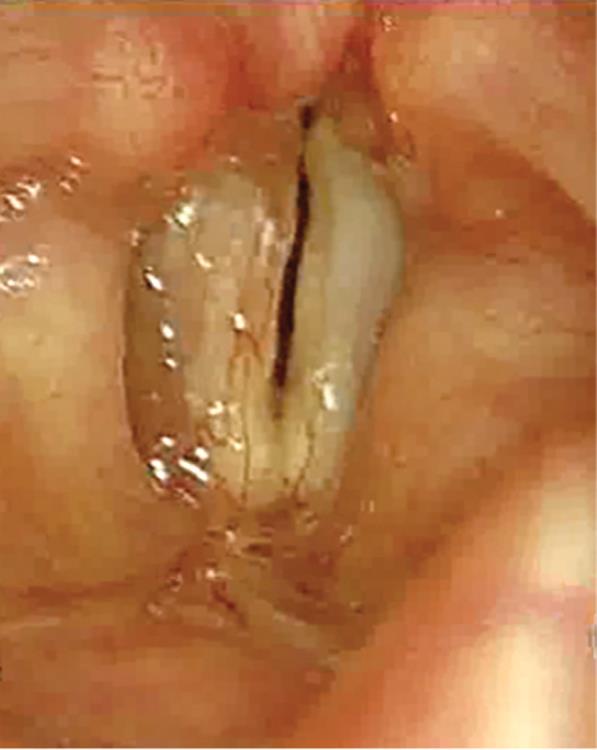
Fig. 1: Stroboscopic picture of patient X giving the appearance of right vocal fold Reinke’s edema with varices
The treatment method for patients depends on the severity of their voice complaints and includes voice therapy and surgical correction. Due to the unpredictable nature of results following the various surgeries described, it is essential to exhaust voice therapy as a beneficial treatment option. The surgical approach is selected casewise and aims to improve glottic efficiency and voice quality by eliminating the restricted vocal cover movement with an optimal glottic closure. These procedures may create epithelial changes, augment the SLP, or medialize the vocal fold.
We describe a surgical technique using the CO2 laser Acublade and performing a release of the sulcus, which is termed as “laser-assisted sulcus release” (LASR).
MATERIALS AND METHODS
A retrospective study was carried out on patients operated for sulcus at our institute over a period of 30 months from February 2017. A documentation of the length and depth of one or more sulci, presence of any associated mucosal bridges, presence of a polyp, cyst or keratin within the sulcus, and any other lesions on the same or opposite vocal fold were noted during microlaryngeal surgery. These sulcus cases were then categorized based on our proposed classification as follows:
- Type A: Single sulcus along the length of half or more of the membranous vocal fold with a depth into the SLP
- Type B: Single sulcus along the length of half or more of the membranous vocal fold with a depth into the ligament (intraligamental sulcus)
- Type C: Single sulcus along the length of half or more of the membranous vocal fold with a depth into the muscle (intramuscular sulcus)
- Type D: Focal pit with a length less than half the membranous vocal fold length and a depth into the SLP
- Type E: Focal pit with a length less than half the membranous vocal fold length and a depth into the ligament (intraligamental focal pit)
- Type F: Focal pit with a length less than half the membranous vocal fold length and a depth into the muscle (intramuscular focal pit)
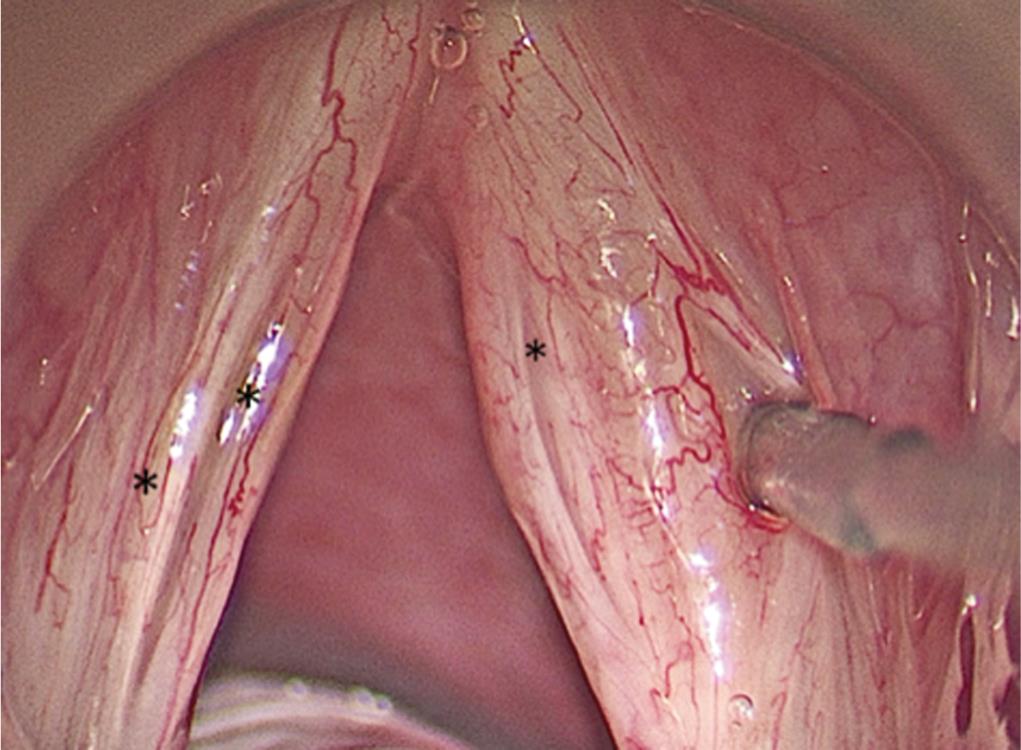
Fig. 2: Microlaryngoscopic view of the vocal folds of patient X revealing a right type A sulcus (*) as well as a right type D sulcus, which is being retracted by a blunt vocal cord dissector. Also observed are two superficial sulci (*) on the left vocal fold (left type A sulci)
In case multiple sulci are present, the number is denoted along with the type. The presence of mucosal bridges may be denoted as MB followed by the number of bridges in brackets; the presence of keratin, cyst, or polyp within a focal pit may be denoted as (K), (C), or (P), respectively. Using this proposed classification, Figure 3 reveals a right type A(P) sulcus and a left type A sulcus.
The second part of our study was an evaluation of the vocal outcomes following LASR. The cases selected for this study were patients with hoarseness with isolated sulcus, which had a depth of invagination into the SLP, where any other associated lesion, such as a mucosal bridge, was not operated on. Thus type A and D sulci were included for the LASR procedure.
Steps of LASR Surgery
Under general anesthesia and microscopic magnification, palpation was followed by subepithelial infiltration using 1:10,000 saline adrenaline solution to elevate the epithelium of the sulcus from its bed. Surgery was performed using the CO2 Acublade laser system with settings of 10 watts power, 0.5 mm length, depth of 1 with time on 0.03 seconds, time off 0.45 seconds in superpulse mode. Using this Acublade, 0.5 mm cuts were made at 90 degrees to the sulcus on its medial and lateral lips keeping intact epithelium of 0.2–0.5 mm in-between these cuts. The estimation of the depth of the sulcus was further confirmed following these epithelial cuts (Fig. 4).
All patients received extensive preoperative therapy, 7–10 days of postoperative voice rest, followed by voice therapy, which continued for a minimum of 3 months.
Preoperative and 3-month postoperative flexible and rigid stroboscopy, GRBAS, and maximum phonatory time (MPT) were compared.
RESULTS
In the study duration, 339 patients were operated for benign glottic lesions and a total of 47 vocal folds with sulci in 36 patients (10.61% patients) were identified, wherein 25 patients had unilateral sulci and 11 patients had bilateral sulci, with or without associated lesions. However, only 18 (38.3%) vocal folds with sulci were diagnosed by preoperative stroboscopy and 29 (61.7%) vocal folds with sulci were incidentally diagnosed during surgery. A total of 56 sulci were diagnosed during our study period and their incidence according to various types is tabulated in Table 1. Laser-assisted sulcus release was performed frequently on patients who had coexisting cysts or polyps, which were excised. However, these cases were not included in the second part of our study and only patients operated by LASR for just their sulci (type A and D) were included (7 patients) and evaluated for the results of the LASR technique. This was in order to eliminate the improvement of vocal outcomes due to the excision of associated lesions. This second part of the study included five patients with bilateral sulci and two patients with unilateral sulci. A total of nine type A sulci and five type D sulci were operated by LASR. All the seven patients who underwent the LASR surgery were females with a mean age of 23.6 years. Preoperative MPT ranged from 2 to 13 seconds with an average of 7.4 seconds and an average GRBAS score of 11.28, while postoperative MPT ranged from 7 to 18 seconds with an average of 13.1 seconds and average GRBAS score of 3.71, which were both found to be statistically significant (p < 0.05). Preoperative stroboscopy revealed a reduced amplitude of mucosal wave in five patients and an almost absent mucosal wave in two patients. Three-month postoperative stroboscopy revealed an improved or normal amplitude with symmetry of mucosal waves and a reduced ventricular hyperadduction. Phonatory gap was observed in all seven patients preoperatively (Fig. 5), which was found to be absent in three and reduced in four patients (Fig. 6) postoperatively.

Fig. 3: Microlaryngoscopic view of vocal folds revealing right type A(P) and left type A sulcus
| Type of sulcus | Number | Associated lesions on same vocal fold | Associated lesions on opposite vocal fold |
|---|---|---|---|
| A (superficial sulcus) | 37 | 3 (P) and 2 P | 1 P |
| 3 MB | 9 C | ||
| 2 (C) | 1 MB | ||
| 1 type D | |||
| B (intraligamental sulcus) | 4 | 1 MB | 1 C |
| 1 P | |||
| C (intramuscular sulcus) | 0 | — | — |
| D (superficial focal pit) | 13 | 3 MB | 2 P |
| 1 P | 1 MB | ||
| 2 (P) | 2 C | ||
| 2 C | |||
| E (intraligamental focal pit) | 2 | 1 (K) | 1 MB |
| 1 P | |||
| F (intramuscular focal pit) | 0 | — | — |
(P), polyp within the sulcus; (C), cyst within the sulcus; (K), keratin within the sulcus; P, polyp; C, cyst; MB, mucosal bridge

Fig. 4: LASR surgery: in a patient with bilateral type A sulci (black asterix), multiple small but complete epithelial laser incisions have been made perpendicular to the lips of the sulci on the left vocal fold and the laser beam (red line) can be seen making an epithelial cut in the median lip of the medial sulcus over the right vocal fold

Fig. 5: Preoperative stroboscopic picture of patient Y during adduction of vocal folds revealing a large spindle-shaped phonatory gap with the bilateral sulcus appreciated
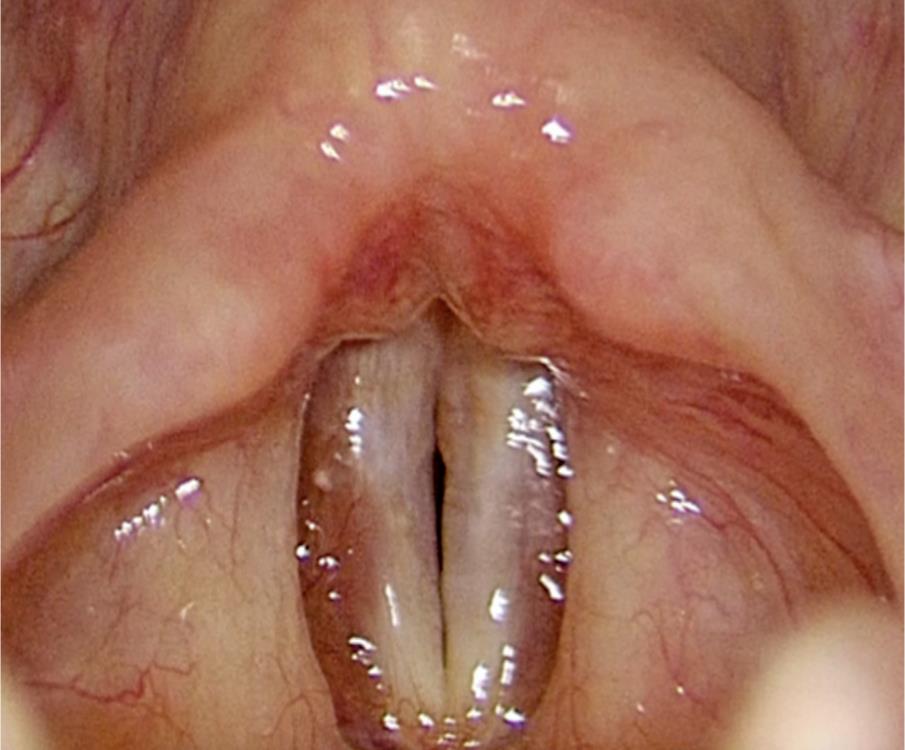
Fig. 6: A 3-month postoperative stroboscopic picture of patient Y revealing a minimal phonatory gap during adduction
DISCUSSION
Difficulties in the definition of sulcus, its pathophysiology, identification, and treatment have prevented optimal management. Dailey and Zeitels noted that SV was the single most unidentified benign glottic entity in a population of patients who were evaluated for benign glottic pathology. Given that the vector of examination is tangent to the site of pathology, there can be an “umbrella effect” that makes identification difficult.17 This visual dilemma makes intraoperative examination that much more important in the identification of lesions such as sulci and mucosal bridges.18 According to Dailey and Zeitels17 study, suspension microlaryngoscopy revealed 16 additional lesions in 9 out of 100 patients. In our study, out of the total 47 vocal folds with sulci, 29 were identified during microlaryngoscopy. Microlaryngeal surgeries should routinely be preceded by careful palpation as this often provides information regarding the location and extent of various glottic pathologies such as sulci and mucosal bridges.
Classification systems described in the past for sulcus have usually been based on stroboscopic examination. One of the earliest classifications given by Bouchayer et al.5 helped us to get introduced to the concept of classifying sulci. They described a SV as one corresponding to an open epidermoid cyst with thickened epithelium with the bottom of the cystic pouch adherent to the vocal ligament and a sulcus vergeture corresponding to atrophy of the mucosa covering the vocal ligament. Nakayama et al.8 classified it as a type I if the sulcus was superficial and confined to SLP, type IIa in which the sulcus was a deep type approximating the vocal ligament, and type IIb as a pouch type. Ford et al.15 in 1996 categorized sulcus into three types. Type I or physiological sulcus is where the depression does not reach the vocal ligament and can be distinguished clinically by noting preservation of vocal fold vibratory activity on videostroboscopy. The presence of mucosal wave activity indicates adequate functional separation of the vocal fold body and cover as described by Hirano and Kakita.19 Type II is described as a full-length depression up to or deeper to the vocal ligament, and type III was deep focal indentation on the vocal fold not involving its entire length. Pontes et al.20 described occult sulcus, sulcus striae minor, sulcus striae major, and sulcus pocket. Sulcus striae (vergeture) is of two types according to the distance between the lips of the sulcus, minor and major. Most classifications do not detail the exact length or depth of the sulcus as this cannot be accurately assessed by stroboscopy. As the length and depth of the sulcus is inversely proportionate to the vocal outcomes following surgery, it is of paramount importance to have a classification system that includes a layered grading of the depth and accurate estimation of the length of the sulcus in order to achieve standardization of results across centers. Keeping this in mind, we have proposed a classification that details the depth as well as length of the sulcus under microscopic magnification following palpation and subepithelial infiltration. In this classification, the number of sulci, presence of associated mucosal bridges, and presence of keratin debris, cysts, or polyps is noted. When the polyp, cyst, or keratin is present within the sulcus, it is labeled in brackets.
A type A sulcus was the commonest type seen in our study wherein a single type A was observed in 19 vocal folds and two type A sulci were observed in 9 vocal folds (i.e., total 37 type A sulci). Type A sulci are often phonotraumatic in origin and may be associated with lesions such as varices, cysts, or polyps on the same or opposite vocal fold. Multiple superficial sulci are quite frequently seen in our practice, unlike the deeper sulci that are usually solitary. There may be associated mucosal bridges, which may be complete or incomplete.18 A type B sulcus was seen in 4/56 sulci; however, a type C sulcus was not seen during the study duration. Type D was the second most common type of sulcus identified in our study and was found in 13/56 sulci. Type E was seen in 2/56 sulci and no type F sulci were identified during our study duration. The majority of patients had a type A sulcus (66.1%) followed by type D (23.2%). Type C and F are extremely rare, usually congenital, and though none were documented in our study duration, they have been identified outside of the study period at our Institute. The deeper sulci and pits (type B, C, E, and F) are rarely associated with secondary lesions on the same or opposite vocal fold due to lack of sufficient glottic closure.16 In our study, superficial sulci (A and D) were associated with other possibly phonotraumatic lesions in 34/50 sulci (68%) as compared to deeper sulci (B, C, E, and F) in 3/6 (50%) cases.
Our classification does not include physiological sulcus as, in a symptomatic patient, even when the sulcus is found incidentally during surgery for another lesion, we cannot be certain that the sulcus is noncontributary toward the hoarseness. A tabulation of the various sulcus classifications is given in Table 2.
Out of the multiple treatment modalities described for sulcus, there is no single method that gives predictable vocal outcomes always. Voice therapy is targeted to improve the incorrect compensatory techniques adopted by these patients, which develops due to glottic insufficiency and poor mucosal waves. The goals of therapy are to encourage vocal hygiene, avoid hyperfunctional activity, and improve the forward focus of voice. It involves relaxation therapy that includes traditional voice therapy techniques, physiological and resonance exercises, and Lax-Vox exercises followed by strengthening exercises done using semioccluded vocal tract exercises at various scales to reduce the phonatory gap. Often even after rigorous long-term therapy, the vocal outcome results may wax and wane. However, it is important to exhaust voice therapy as a treatment modality prior to consideration of surgery as the results of surgery are also unpredictable.
The aim of most surgical techniques is to decrease the phonatory gap and improve the mucosal wave. Hirano’s21 study on the histological architecture of the vocal folds and the cover-body theory emphasized that the motion ability of the cover over the muscle is essential for normal vocal quality. The surgical methods may be broadly divided as epithelial surgeries, augmentation of SLP, and medialization techniques.
| Bouchayer et al. | Nakayama et al. | Ford et al. | Pontes et al. | Authors |
|---|---|---|---|---|
| — | Type I | Type I | Occult sulcus | — |
| Sulcus vergeture | Type II | Stria minor | A (superficial sulcus) | |
| Type IIa | Stria major | B (intraligamental sulcus) | ||
| — | — | C (intramuscular sulcus) | ||
| Sulcus vocalis | Type IIb | Type III | Sulcus pocket | D (superficial focal pit) |
| E (intraligamental focal pit) | ||||
| — | F (intramuscular focal pit) |
Sulcussectomy described by Hirano,22 the sliding down technique by Saito,23 and partial sulcussectomy by Roch et al.24 are some examples of epithelial surgeries. Ford and colleagues15 noted variable improvement of stroboscopic parameters following cold instrument excision of sulcus for patients who have Ford type III sulcus. Undermining of sulcus with cold instruments or laser has also been described followed by redraping of the epithelium without suturing or gluing.15
The slicing mucosa technique was described by Pontes and Behlau25 with the purpose of interrupting the fibrotic and tension lines of the sulcus to yield a free mucosal vibration by using the principles of scar contracture repair. A parallel incision is made 1 mm above the superior lip of the sulcus and mucosa is detached without touching the vocal ligament. Multiple incisions of different lengths are made in the detached mucosa perpendicular to the sulcus, producing mucosal slices that are positioned without suturing.
Medialization of the vocal fold may be performed by external thyroplasty for closure of the glottal gap,26 by injecting fat into the paraglottic space27 or by augmentation of SLP by using various materials. Fat implantation into the SLP was reported by Sataloff and colleagues28 in 1997 and can also be used as an adjunctive procedure with medialization thyroplasty, fat injection into the paraglottic space, scar excision, and steroid injection.29 Tsunoda and colleagues30–32 reported on the use of temporalis fascia as a superficial and deep implant into the vocal fold. Ford introduced Alloderm strips longitudinally within Reinke’s space to restore pliability and tissue loss within the SLP.33 Work with implantation of stem cells and fascial implant suggests that the host tissues replace the implants over time, which helps to explain the vocal variability.34,35 Office-based injection laryngoplasty using materials such as collagen, hyaluronic acid, or hydroxyapetite is a relatively safe and effective method of treatment of glottic insufficiency.36 The physical injection of collagen aids in the closure of the glottic gap; it is postulated to alter the nature of the adjacent scar and makes it more pliable by lamina propria remodeling.37 Ford and Bless38 noted that this modality is best applied for more modest gaps, and that large defects are not addressed well with this approach. Prospective studies conducted by Welham et al. showed that there is no single effective method of treatment and that there is no evidence-based decision algorithm for matching a given treatment to a given patient.39
Laser-assisted sulcus release surgery is based on principles of the slicing mucosa technique as well as the principle underlying radial incisions for subglottic stenosis with a CO2 laser.40 By making multiple small but complete epithelial incisions on the overlying epithelium of the medial and lateral lips of superficial sulci and focal pits (type A and D), the aim is to break the fibrous bands and release the tension enough to flatten the sulcus and allow normal epithelium to grow into the incised raw portions that are created between interspersed normal mucosa. The epithelium is not elevated from its bed in this technique in order to avoid increasing inflammation and scarring during the recovery phase. Cold instruments were tried for this technique but the CO2 Acublade laser was found to be more precise. Postoperatively, a week of voice rest is followed by relaxation therapy for 4–6 weeks followed by a repeat stroboscopy prior to introducing strengthening and pitch exercises for 3 months. The 3-month postoperative stroboscopy revealed a reduced phonatory gap with an increased amplitude of the mucosal wave; however, this did not always result in an optimal voice. In contrast to this, occasionally the postoperative vocal outcomes were greatly improved although stroboscopy revealed a minimal phonatory gap and a gentle persistence of the sulcus. This unpredictable relationship between vocal outcome measures and stroboscopy remains unexplained and needs further evaluation.
CONCLUSION
Microlaryngeal examination with palpation of the vocal folds revealed a sulcus in 61.7% of stroboscopically undiagnosed lesions. The incidence of types of sulci found was 66.1% type A, 7.1% type B, 23.2% type D, 3.6% type E, and no type C or F during the study duration. LASR in our limited series of seven patients (14 sulci in 10 vocal folds) revealed a significant improvement in MPT and GRBAS; however, this did not always match the stroboscopy picture. This is a pilot study of 14 sulci managed with LASR and more work is needed in this area to make a definitive conclusion.
CLINICAL SIGNIFICANCE
Proposed classification system and surgery have shown promising results and should be further studied.
REFERENCES
1. Giacomini C. Report on the anatomy of the negro (in Italian). Acad Med Torino 1892;40:17–61.
2. Dailey SH, Ford CN. Surgical management of sulcus vocalis and vocal fold scarring. Otolaryngol Clin North Am 2006;39(1):23–42. DOI: 10.1016/j.otc.2005.10.012.
3. Miaśkiewicz B, Szkiełkowska A, Piłka A, et al. Results of surgical treatment in patients with sulcus vocalis. Otolaryngol Pol 2015;69(6):7–14. DOI: 10.5604/00306657.1182613.
4. Arnold GE. Dysplastic dysphonia: minor anomalies of the vocal cords causing persistent hoarseness. Laryngoscope 1958;68(2):142–158. DOI: 10.1288/00005537-195802000-00006.
5. Bouchayer M, Cornut G, Witzig E, et al. Epidermoid cysts, sulci, and mucosal bridges of the true vocal cord: a report of 157 cases. Laryngoscope 1985;95(9 Pt 1):1087–1094. DOI: 10.1288/00005537-198509000-00014.
6. Sato K, Hirano M. Electron microscopic investigation of sulcus vocalis. Ann Otol Rhinol Laryngol 1998;107(1):56–60. DOI: 10.1177/000348949810700111.
7. Husain S, Sulica L. Familial sulcus vergeture: further evidence for origin of type II sulcus. J Voice 2016;30(6):761.e19–761.e21. DOI: 10.1016/j.jvoice.2015.10.001.
8. Nakayama M, Ford CN, Brandenburg JH, et al. Sulcus vocalis in laryngeal cancer: a histopathologic study. Laryngoscope 1994;104(1 Pt 1):16–24. DOI: 10.1288/00005537-199401000-00005.
9. Giovanni A, Chanteret C, Lagier A. Sulcus vocalis: a review. Eur Arch Otorhinolaryngol 2007;264(4):337–344. DOI: 10.1007/s00405-006-0230-8.
10. Van Caneghan D. The etiology of the vocal cord furrow (in French). Ann Mal Oreille Larynx Nez Pharynx 1928;43:121–130.
11. Priston J. The evolution of an epidermoid cyst during vocal mutation (in Portuguese). In: Behlau M, ed. O Melhor Que Vi e Ouvi Atualizaçãoem Laringologia e Voz. Revinter: Rio de Janeiro; 1998. pp. 114–120.
12. Itoh T, Kawasaki H, Morikawa I, et al. Vocal fold furrows. A 10 year review of 240 patients. Auris Nasus Larynx 1983;10 (Suppl):S17–S26. DOI: 10.1016/S0385-8146(83)80002-9.
13. Byeon HK, Kim JH, Kwon JH, et al. Clinical characteristics of vocal polyps with underlying sulcus vocalis. J Voice 2013;27(5):632–635. DOI: 10.1016/j.jvoice.2013.04.010.
14. Nerurkar N, Gupta H, Shedge A. Diagnostic challenge of sulcus vocalis made easier. Int J Phonosurg Laryngol 2015;5(2):39–41. DOI: 10.5005/jp-journals-10023-1102.
15. Ford CN, Inagi K, Khidr A, et al. Sulcus vocalis: a rational analytical approach to diagnosis and management. Ann Otol Rhinol Laryngol 1996;105(3):189–200. DOI: 10.1177/000348949610500304.
16. Pontes P, Behlau M. Sulcus mucosal slicing technique. Curr Opin Otolaryngol Head Neck Surg 2010;18(6):512–520. DOI: 10.1097/MOO.0b013e3283402a3b.
17. Dailey SH, Spanou K, Zeitels SM. The evaluation of benign glottic lesions: rigid telescopic stroboscopy vs suspension microlaryngoscopy. J Voice 2007;21(1):112–118. DOI: 10.1016/j.jvoice.2005.09.006.
18. Nerurkar NK, Sapre A, Gosavi R. Mucosal bridges (MB): a 9 year retrospective study of their incidence with a third variant proposed. Eur Arch Otorhinolaryngol 2019;276(1):159–165. DOI: 10.1007/s00405-018-5218-7.
19. Hirano M, Kakita Y. Cover-body theory of vocal fold vibration. In: Daniloff RG, ed. Speech science.San Diego, Calif: College-Hill Press; 1985. pp. 1–46.
20. Pontes P, Behlau M, Gonçalves MIR. Minor structural alterations of the larynx: basic aspects (in Portuguese). Acta AWHO 1994;2:175–185.
21. Hirano M. Morphological structure of the vocal cord as a vibrator and its variation. Folia Phoniatr (Basel) 1974;26:89–94. DOI: 10.1159/000263771.
22. Hirano M. Phonosurgery: basic clinical investigations. Otologia (Fukuoka) 1975;21 (Suppl. 1):239–442.
23. Saito S. Phonosurgery: basics on the mechanism of phonation and endolaryngeal microsurgery. Otologia (Fukuoka) 1977;23 (Suppl. 1):171–386.
24. Roch JB, Bouchayer M, Cornut G. Le sulcus glottidis. Rev Laryngol Otol Rhinol (Bord) 1981;102(7–8):333–346.
25. Pontes P, Behlau M. Treatment of sulcus vocalis: auditory perceptual and acoustical analysis of the slicing mucosa surgical technique. J Voice 1993;7(4):365–376. DOI: 10.1016/S0892-1997(05)80260-7.
26. Netterville JL, Stone RE, Luken ES, et al. Silastic medialization and arytenoid adduction: the Vanderbilt experience. A review of 116 phonosurgical procedures. Ann Otol Rhinol Laryngol 1993;102(6):413–424. DOI: 10.1177/000348949310200602.
27. Hsiung MW, Lin YS, Su WF, et al. Autogenous fat injection for vocal fold atrophy. Eur Arch Otorhinolaryngol 2003;260(9):469–474. DOI: 10.1007/s00405-003-0622-y.
28. Sataloff RT, Spiegel JR, Hawkshaw MJ. Vocal fold scar. Ear Nose Throat J 1997;76(11):776. DOI: 10.1177/014556139707601103.
29. Neuenschwander MC, Sataloff RT, Abaza MM, et al. Management of vocal fold scar with autologous fat implantation: perceptual results. J Voice 2001;15(2):295–304. DOI: 10.1016/S0892-1997(01)00031-5.
30. Tsunoda K, Baer T, Niimi S. Autologous transplantation of fascia into the vocal fold: long term results of a new phonosurgical technique for glottal incompetence. Laryngoscope 2001;111(3):453–457. DOI: 10.1097/00005537-200103000-00014.
31. Tsunoda K, Niimi S. Autologous transplantation of fascia into the vocal fold. Laryngoscope 2000;110(4):680–682. DOI: 10.1097/00005537-200004000-00026.
32. Tsunoda K, Takanosawa M, Niimi S. Autologous transplantation of fascia into the vocal fold: a new phonosurgical technique for glottal incompetence. Laryngoscope 1999;109(3):504–508. DOI: 10.1097/00005537-199903000-00030.
33. Welham NV, Rousseau B, Ford CN, et al. Perceptual, instrumental, and psychosocial outcomes after phonosurgery for sulcus vocalis. Otolaryngol Head Neck Surg 2004;131(2):P257. DOI: 10.1016/j.otohns.2004.06.527.
34. Kanemaru S, Nakamura T, Omori K, et al. Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol 2003;112(11):915–920. DOI: 10.1177/000348940311201101.
35. Hsiung MW, Kang BH, Pai L, et al. Combination of fascia transplantation and fat injection into the vocal fold for sulcus vocalis: long-term results. Ann Otol Rhinol Laryngol 2004;113(5):359–366. DOI: 10.1177/000348940411300504.
36. Ford CN, Bless DM, Campbell D. Studies of injectable soluble collagen for vocal fold augmentation. Rev Laryngol Otol Rhinol (Bord) 1987;108(1):33–36.
37. Ford C, Bless DM. Collagen injection in the scarred vocal fold. J Voice 1987;1:116–118. DOI: 10.1016/S0892-1997(87)80034-6.
38. Remacle M, Lawson G, Degols JC, et al. Microsurgery of sulcus vergeture with carbon dioxide laser and injectable collagen. Ann Otol Rhinol Laryngol 2000;109(2):141–148. DOI: 10.1177/000348940010900206.
39. Welham NV, Choi SH, Dailey SH, et al. Prospective multi-arm evaluation of surgical treatment for vocal fold scar and pathologic sulcus vocalis. Laryngoscope 2011;121(6):2152–2160. DOI: 10.1002/lary.21780.
40. Shapshay SM, Beamis JF,Jr Hybels RL, et al. Endoscopic treatment of subglottic and tracheal stenosis by radial laser incision and dilation. Ann Otol Rhinol Laryngol 1987;96(6):661–664. DOI: 10.1177/000348948709600609.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.